The adaptor protein 14-3-3ζ is encoded by the yhwaz gene and implicated in a variety of organic processes.
In tumorigenesis, 14-3-3ζ acknowledges particular phosphorylation motifs and interacts with a whole lot of goal proteins and is, thus, concerned in the regulation of tumor proliferation, migration and differentiation.
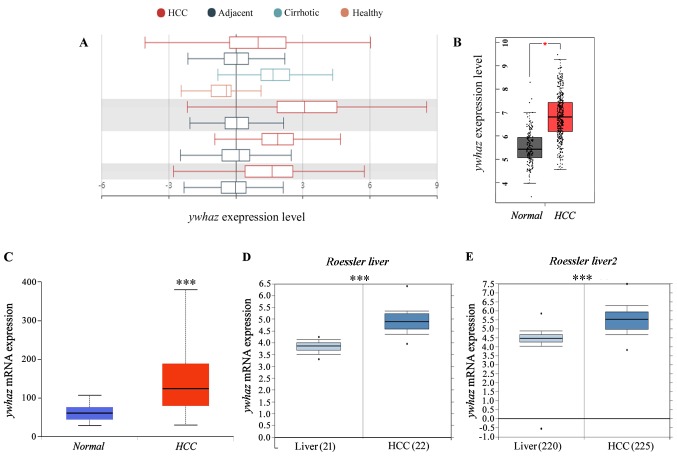
In the current research, bioinformatics instruments had been used to research knowledge from The Cancer Genome Atlas and Gene Expression Omnibus databases and the expression of yhwaz, and gene regulation networks had been recognized as doubtlessly related in hepatocellular carcinoma (HCC).
In HCC, yhwaz expression was demonstrated to be upregulated and considerably related to poor prognosis. Expression ranges of microRNAs focusing on yhwaz had been related to improved prognosis in sufferers with liver most cancers.
Gene networks which might be regulated by yhwaz had been discovered to be concerned in cell cycle regulation and tumorigenesis, indicating the potential use of the expression ranges of yhwaz in liver tissue as predictive biomarkers in sufferers with liver most cancers.
In the current research, yhwaz was recognized as a gene of curiosity by knowledge mining gene expression databases and its involvement in regulatory networks in HCC was indicated.
Therefore, additional in vitro and in vivo research on the position of yhwaz in the carcinogenesis of HCC could be drastically helpful.
CRIF1 overexpression facilitates tumor development and metastasis by inducing ROS/NFκB pathway in hepatocellular carcinoma.
CR6-interacting issue 1 (Crif1) is a mitochondrial protein which is required for the meeting of oxidative phosphorylation (OXPHOS) complexes. Our bioinformatics evaluation primarily based on Cancer Genome Atlas (TCGA) database revealed an aberrant overexpression of CRIF1 in hepatocellular carcinoma (HCC).
However, the medical significance and organic features of CRIF1 are nonetheless unclear in this malignancy. Here, we report that CRIF1 is continuously overexpressed in HCC cells primarily because of the downregulation of miR-497-5p, which is related to poor prognosis of sufferers with HCC.
CRIF1-promoted HCC development and metastasis by suppressing cell apoptosis and inducing cell cycle development and epithelial to mesenchymal transition (EMT).
Mechanistically, elevated mitochondrial ROS manufacturing and consequently activation of the NFκB signaling pathway was discovered to be concerned in the promotion of development and metastasis by CRIF1 in HCC cells.
In abstract, CRIF1 performs an oncogenic position in HCC development by activating ROS/NFKB pathway, implying CRIF1 as a possible prognostic issue and therapeutic goal in HCC.
